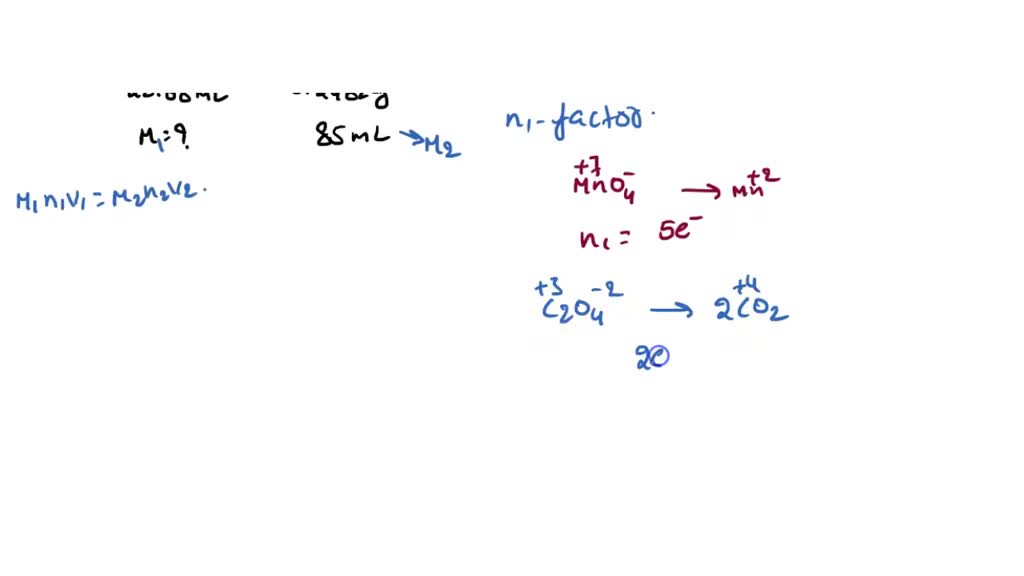
Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) . Express your answer as an ion. titration of an acidic solution of na2c2o4 with kmno4(aq). Which species is reduced in this reaction? Which species is reduced in this reaction? in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. to evaluate a redox titration we need to know the shape of its titration curve. 30 s) pink color as the end point. titrate with kmno4 at 60~90 oc with stirring. Which species is the reducing. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. The oxidative property of the permanganate ion.
from www.numerade.com
titration of an acidic solution of na2c2o4 with kmno4(aq). Which species is the reducing. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. Which species is reduced in this reaction? in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. 30 s) pink color as the end point. Express your answer as an ion. Which species is reduced in this reaction? The oxidative property of the permanganate ion.
SOLVED KMnO4, can be standardized in Potassium permanganate, acidic
Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) The oxidative property of the permanganate ion. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. titration of an acidic solution of na2c2o4 with kmno4(aq). 30 s) pink color as the end point. Express your answer as an ion. titrate with kmno4 at 60~90 oc with stirring. Which species is reduced in this reaction? Which species is the reducing. in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. Which species is reduced in this reaction? The oxidative property of the permanganate ion. to evaluate a redox titration we need to know the shape of its titration curve. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using.
From www.slideserve.com
PPT REDOX TITRATIONS PowerPoint Presentation ID2670469 Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) titrate with kmno4 at 60~90 oc with stirring. titration of an acidic solution of na2c2o4 with kmno4(aq). to evaluate a redox titration we need to know the shape of its titration curve. Which species is the reducing. The oxidative property of the permanganate ion. if the concentration of the khp solution is known accurately and the. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) The oxidative property of the permanganate ion. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. Which species is reduced in this reaction? to evaluate a redox titration we need to know the shape of its titration curve. titration. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
SOLVED A solution of sodium oxalate (Na2C2O4) in acidic solution is Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) titration of an acidic solution of na2c2o4 with kmno4(aq). Which species is reduced in this reaction? Which species is reduced in this reaction? to evaluate a redox titration we need to know the shape of its titration curve. 30 s) pink color as the end point. in this section, we will see how to perform calculations to. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
SOLVED 0.04M of KMnO4 solution was titrated with 1.00 ml of (13.4 g Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Which species is the. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.scribd.com
Standardization of KMnO4 Solution by Na2C2O4 PDF Titration Chemistry Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) Which species is the reducing. Express your answer as an ion. titration of an acidic solution of na2c2o4 with kmno4(aq). Which species is reduced in this reaction? Which species is reduced in this reaction? if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.chegg.com
Solved H2C2O4 (aq) + 2NaOH (aq) Na2C2O4 (aq) + 2 H20 (1) A Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) Which species is the reducing. 30 s) pink color as the end point. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Which species is reduced in this reaction? The oxidative property of the permanganate ion. Which species is reduced in. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
A solution of sodium oxalate (Na2C2O4) in acidic solution is titrated Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) to evaluate a redox titration we need to know the shape of its titration curve. in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
SOLVED KMnO4 can be standardized in acidic media by using its redox Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) to evaluate a redox titration we need to know the shape of its titration curve. in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. Which species is reduced in this reaction? titration of an acidic solution of na2c2o4 with kmno4(aq). in this section, we will. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
SOLVED A 0.1278 g sample of primary standard Na2C2O4 is diluted to 100 Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. titration of an acidic solution of na2c2o4 with kmno4(aq). Which species is reduced in this reaction? titrate with kmno4 at 60~90 oc with stirring. The oxidative property of the permanganate. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) 30 s) pink color as the end point. Which species is the reducing. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Which species is reduced in this reaction? titrate with kmno4 at 60~90 oc with stirring. if the. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
SOLVED Titration of 20.00 mL of solution containing 6 an unknown Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) titration of an acidic solution of na2c2o4 with kmno4(aq). Which species is the reducing. The oxidative property of the permanganate ion. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. 30 s) pink color as the end point. in. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.sliderbase.com
Titration Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) Which species is reduced in this reaction? Express your answer as an ion. in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. titration of an acidic solution of na2c2o4 with kmno4(aq). Which species is reduced in this reaction? 30 s) pink color as the end point. The. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
SOLVED A student performed the Redox Titration procedure as given in Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) to evaluate a redox titration we need to know the shape of its titration curve. in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. titration of an acidic solution of na2c2o4 with kmno4(aq). The oxidative property of the permanganate ion. if the concentration of the. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.toppr.com
KMnO4 acts as an oxidizing agent in acidic medium. The number of moles Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) Express your answer as an ion. Which species is reduced in this reaction? if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. Which species is reduced in this reaction? in this section, we will see how to perform calculations to. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.slideserve.com
PPT Ch. 20 Electrochemistry PowerPoint Presentation, free download Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. titrate with kmno4 at 60~90 oc with stirring. to evaluate a redox titration we need to know the shape of its titration curve. 30 s) pink color as the end. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.youtube.com
Standardization of KMnO4 by using Na2C2O4 solutionDetermination KMnO4 Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) Which species is the reducing. titrate with kmno4 at 60~90 oc with stirring. in a volumetric analysis experiment, a solution of sodium oxalate (na2c2o4) in acidic solution is titrated with a solution of. to evaluate a redox titration we need to know the shape of its titration curve. 30 s) pink color as the end point. Which. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From slideplayer.com
Unit 4 Solutions Chemistry ppt download Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) Which species is the reducing. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. The oxidative property of the permanganate ion. Express your answer as an ion. 30 s) pink color as the end point. in this section, we will. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).
From www.numerade.com
Experiment No. 6 Standardization of potassium permanganate solution Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq) Which species is reduced in this reaction? Which species is the reducing. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out carefully, then the. Which species is reduced in this reaction? titrate with kmno4 at 60~90 oc with stirring. The oxidative property of. Titration Of An Acidic Solution Of Na2C2O4 With Kmno4(Aq).